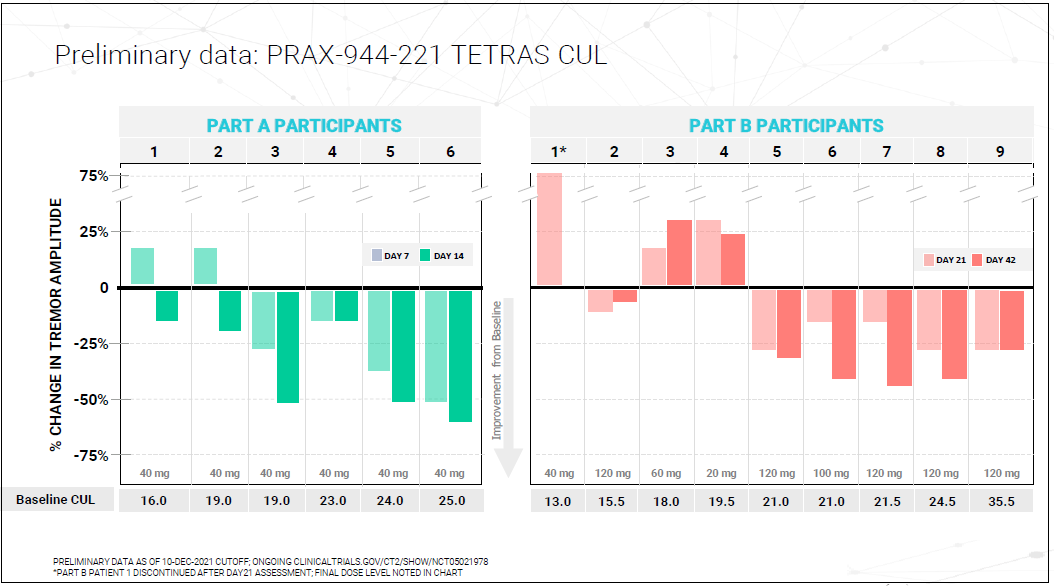
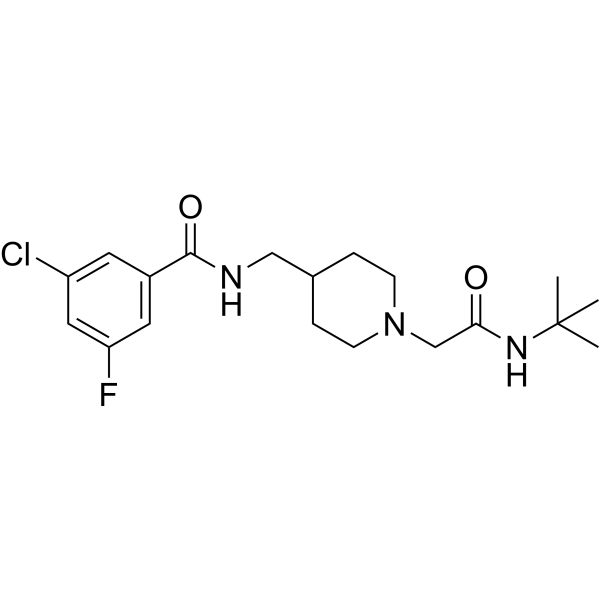
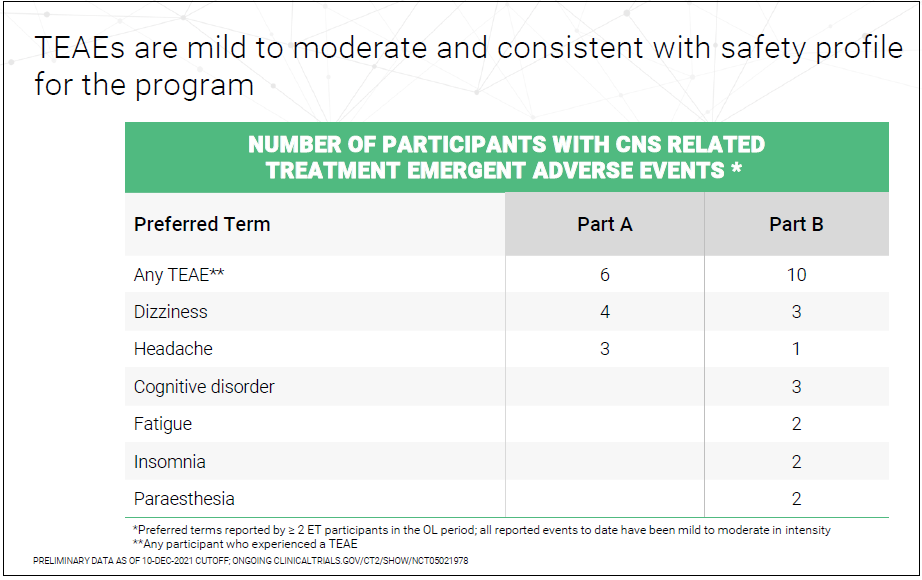
Is Ulixacaltamide Available In Usa - Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the.
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are.
Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits.
Essential Tremor Agent Ulixacaltamide Continues to Show Positive
Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. In our earlier phase.
ulixacaltamide (PRAX944) / Praxis Precision Medicines
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Essential tremor affects up to seven.
Praxis Precision Medicines Announces Topline Results from the
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. Essential tremor affects up to seven million people.
Essential Tremor Agent Ulixacaltamide Continues to Show Positive
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide,.
Essential Tremor Agent Ulixacaltamide Continues to Show Positive
In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Essential tremor affects up to seven.
Praxis Precision Medicines to Present on Ulixacaltamide at the American
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Orphan designation is assigned by a.
Mechanism Behind Ulixacaltamide to Treat Essential Tremor Marcio Souza
In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. Ulixacaltamide is an investigational new drug developed.
ulixacaltamide药品详情NextPharma®数据库ByDrug医药魔方数据库
Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide is.
ulixacaltamide (PRAX944) / Praxis Precision Medicines
Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. In our earlier.
An Innovative Multistudy Phase 3 Program to Evaluate the Efficacy and
Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Orphan designation is assigned by a regulatory body to encourage companies to develop drugs for rare diseases. In our earlier phase.
Orphan Designation Is Assigned By A Regulatory Body To Encourage Companies To Develop Drugs For Rare Diseases.
In our earlier phase 2 essential1 study, we observed that patients who were already on propranolol experienced additional benefits. Ulixacaltamide is an investigational new drug developed by praxis precision medicines, designed to treat essential tremor, [1] a. Essential tremor affects up to seven million people in the united states with action tremors that impact daily living, and there are. Ulixacaltamide, the most advanced program within praxis’ cerebrum™ small molecule platform, is currently in development for the.