When Will Sepranolone Be Available - Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. As of now, it is undergoing phase iii.
Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. As of now, it is undergoing phase iii. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for.
As of now, it is undergoing phase iii. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for.
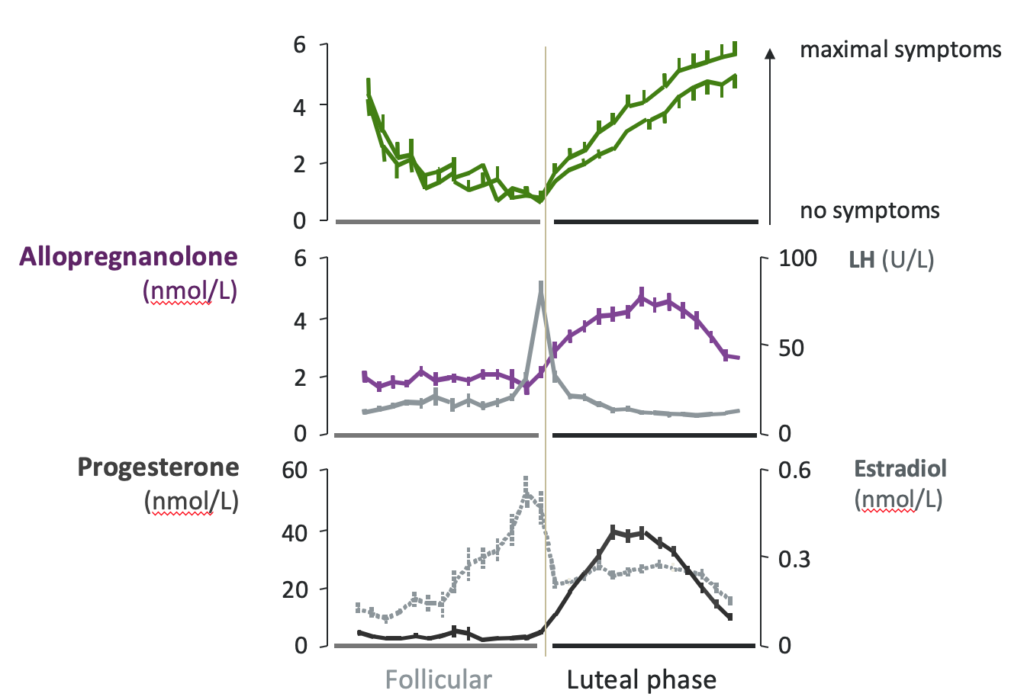
Sepranolone and PMDD Asarina Pharma
As of now, it is undergoing phase iii. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Relmada therapeutics has announced the acquisition of.
(PDF) A randomized, doubleblind study on efficacy and safety of
Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. As of now, it is undergoing phase iii. Relmada purchases sepranolone, a.
SAPHNELO Dosage & Rx Info Uses, Side Effects
As of now, it is undergoing phase iii. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Under the terms of the agreement, relmada acquires all rights.
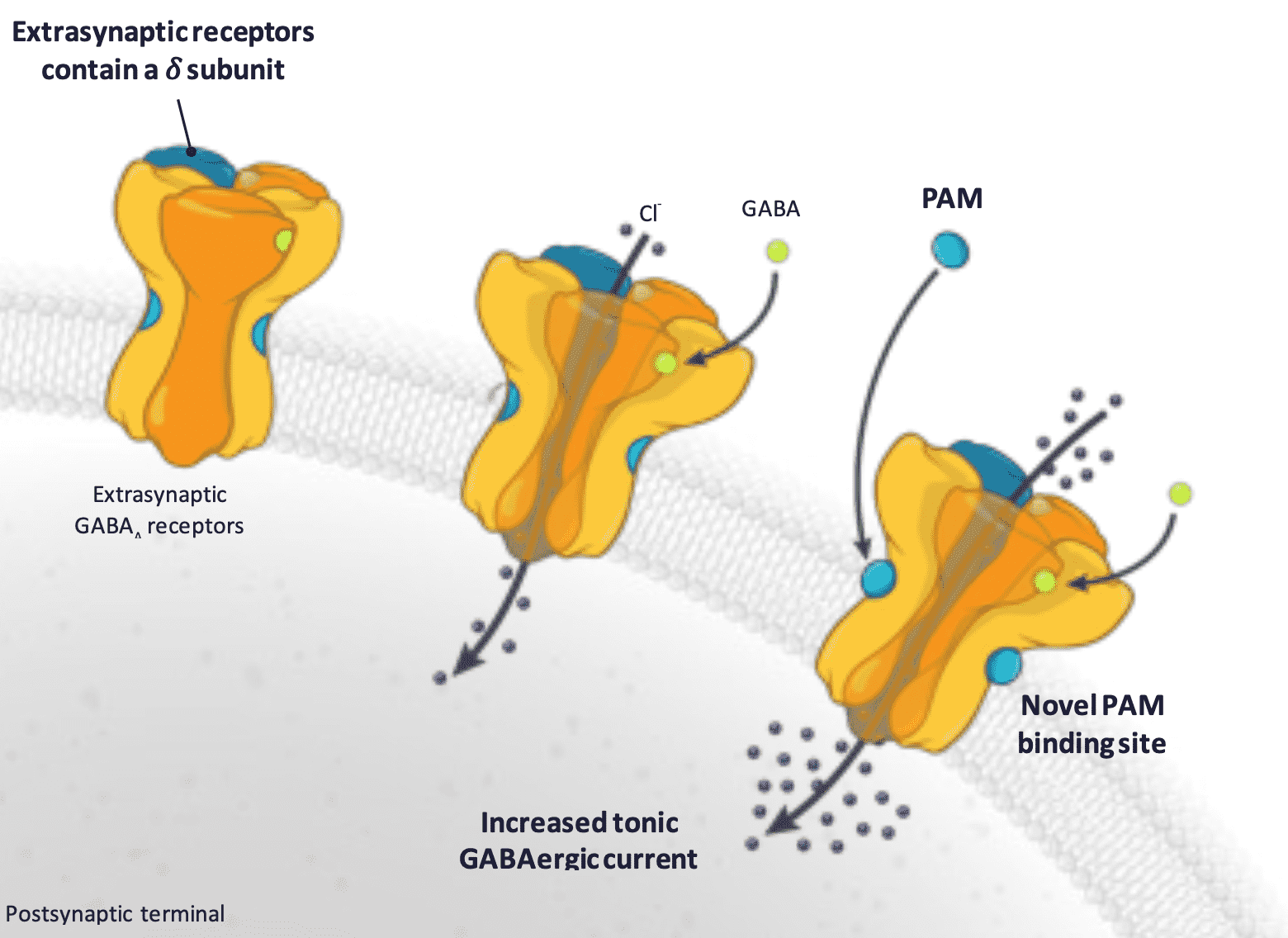
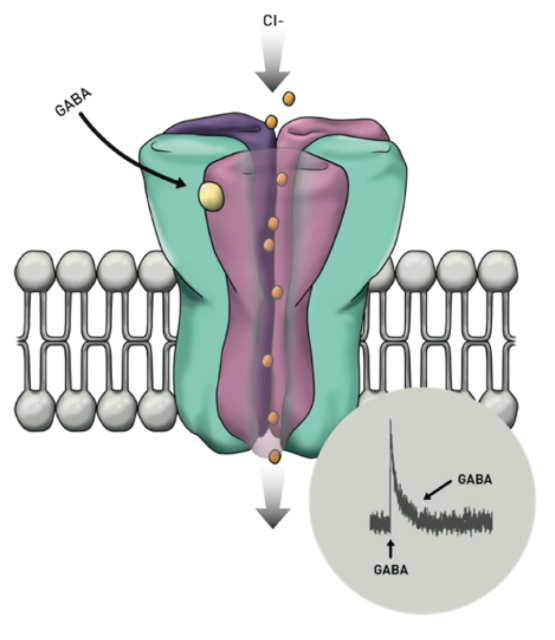
How does Sepranolone work? Asarina Pharma
Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals..
Table 1 from A randomized, doubleblind study on efficacy and safety of
Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. As of now, it is undergoing phase iii. Relmada therapeutics has announced the acquisition.
Sepranolone and Tourette Asarina Pharma
Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. As of now, it is undergoing phase iii. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development..
Sepranolone and PMDD Asarina Pharma
Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. As of now, it is undergoing phase iii. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Sepranolone’s availability is contingent.
Change in Sum21 during nine luteal phase days from baseline to the 3rd
Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Sepranolone’s availability is contingent upon the completion of clinical.
Pin på PMDD
Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. As of now, it is undergoing phase iii. Sepranolone’s.
Figure 2 from Treatment of premenstrual dysphoric disorder with the
Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals. Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada therapeutics, inc., a biotechnology company in the clinical development phase, has announced the. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome.
Relmada Therapeutics, Inc., A Biotechnology Company In The Clinical Development Phase, Has Announced The.
Under the terms of the agreement, relmada acquires all rights to sepranolone, enabling it to continue the clinical development. Relmada purchases sepranolone, a phase 2b ready asset, for the treatment of tourette syndrome (ts) and other compulsion. Relmada therapeutics has announced the acquisition of sepranolone, a phase 2b ready neurosteroid developed for. Sepranolone’s availability is contingent upon the completion of clinical trials and regulatory approvals.