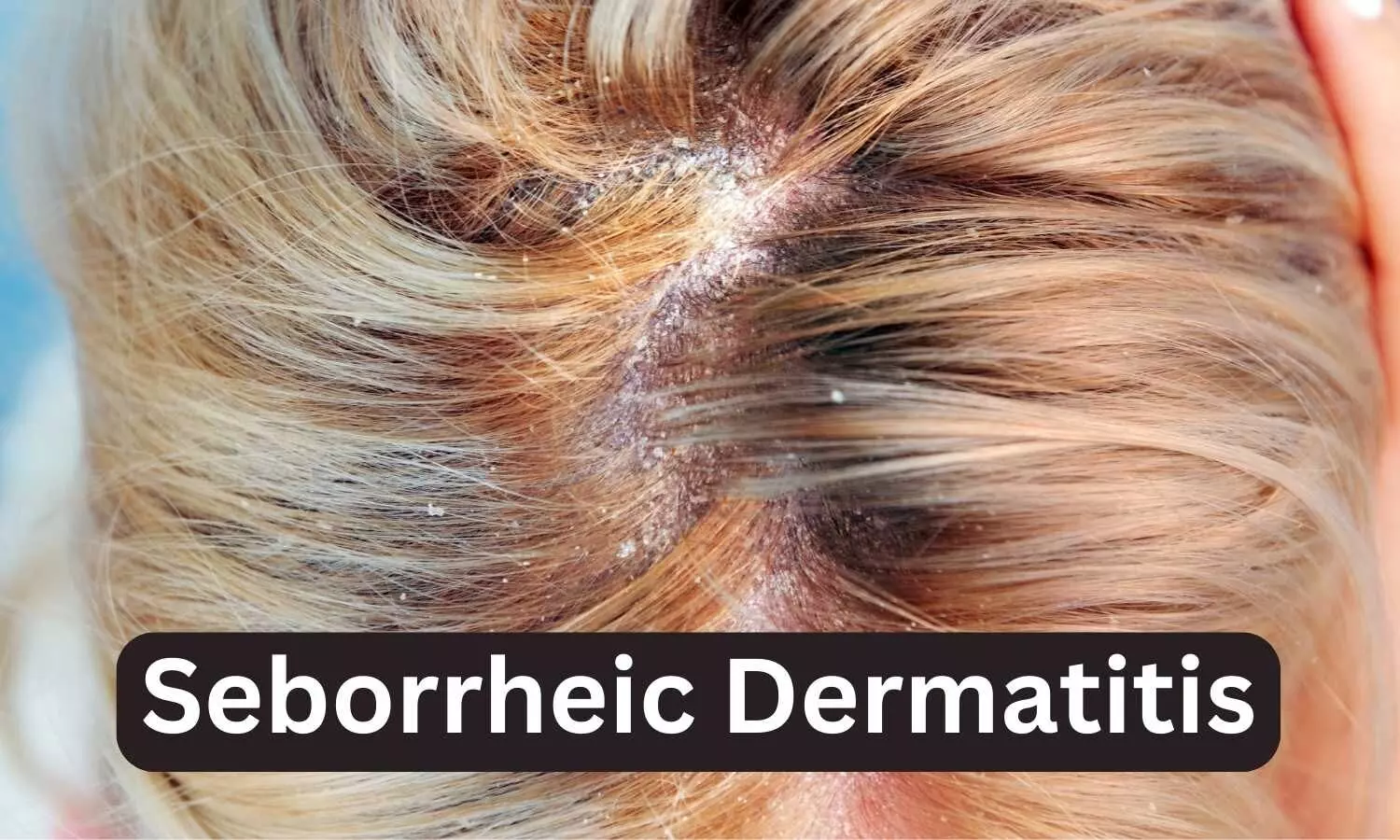
When Will Roflumilast Foam Be Available - On may 22, 2025, the fda approved roflumilast (zoryve; The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key.
Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. On may 22, 2025, the fda approved roflumilast (zoryve;
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and.
ZORYVE® (roflumilast) topical foam, 0.3 Patient Website
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Arcutis biotherapeutics) topical foam 0.3%.
Roflumilast
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Food and drug administration has approved zoryve (roflumilast) topical foam.
ZORYVE (roflumilast) topical foam 0.3 Official HCP Website
Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today.
FDA Approves Arcutis’ ZORYVE® (roflumilast) Cream 0.3 for Treatment of
Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic.
ZORYVE® (roflumilast) topical foam, 0.3 Arcutis Biotherapeutics
On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. The 0.3% foam formulation.
Roflumilast topical foam receives FDA approval for treating seborrheic
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. On may 22, 2025, the fda approved roflumilast (zoryve; Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Zoryve foam 0.3% is.
Roflumilast Tablets Solco Healthcare
On may 22, 2025, the fda approved roflumilast (zoryve; Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as.
ZORYVE® (roflumilast) topical foam, 0.3 Patient Website
Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. On may 22, 2025, the fda approved roflumilast (zoryve; The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Food and.
DailyMed ZORYVE roflumilast aerosol, foam
Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. The 0.3% foam formulation.
FDA Approves Arcutis’ ZORYVE® (roflumilast) Topical Foam, 0.3 for the
Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Zoryve foam 0.3% is.
Food And Drug Administration Has Approved Zoryve (Roflumilast) Topical Foam 0.3 Percent For The Treatment Of.
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new.