Spi-1005 Availability - A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
Jonathan Kil, MD, PhD. "Development of SPI1005 for Hearing Loss and
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
Sound Pharmaceuticals to launch Covid19 oral therapeutic trial
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
SPI1005 Middle Dose for Hearing Loss Clinical Trial 2024 Power
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
SOUND PHARMA 完成了 SPI1005 治疗梅尼埃病(涉及听力损失、耳鸣和头晕)的关键 3 期临床试验的注册 知乎
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
SPI1005 for Meniere's Disease Clinical Trial 2024 Power
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
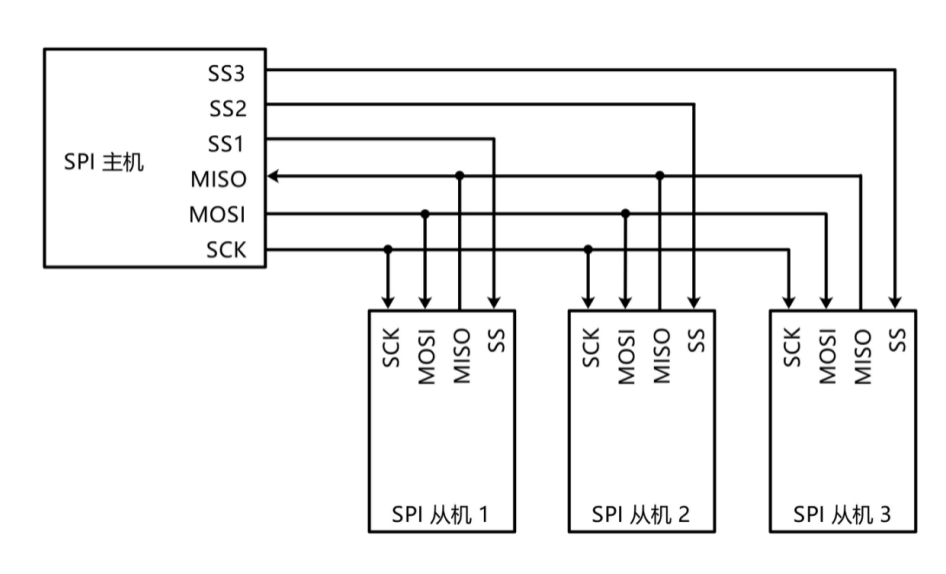
SPI通信协议_spi上拉CSDN博客
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
Be Patient spi 1005 is still in trials YouTube
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
Phase 3 for Ebselen SPI 1005 has started r/tinnitusresearch
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.
Development of SPI1005 for Hearing Loss and Tinnitus Jonathan Kil
A total of 254 patients were screened for eligibility at 11 sites, including leading academic centers in the u.s.