Merck Clinical Trial Recruitment Strategies Recent - We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada).
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective.
Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial.
Patient Recruitment for Clinical Trials Key Challenges, Optimization
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require four phases, in addition to government review.
10 Clinical Trial Recruitment Strategies That Work
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Awareness of challenges.
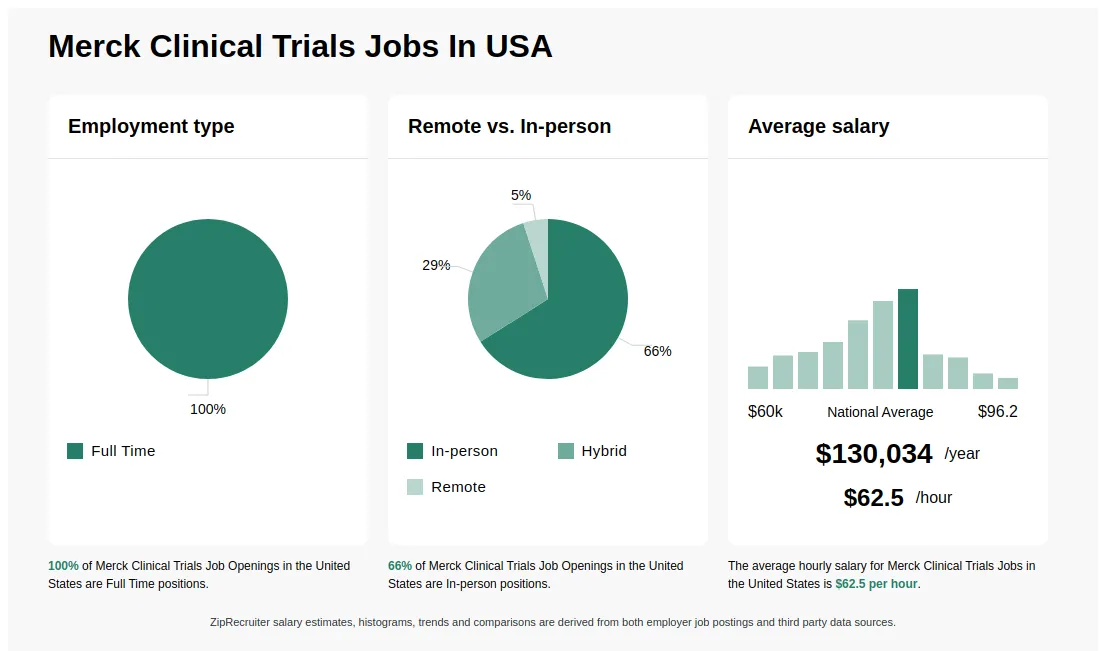
5083/hr Merck Clinical Trials Jobs (NOW HIRING) Feb 2025
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Clinical trials require four phases, in addition to government.
Merck Keynote Clinical Trial Branding by Melissa Sieja at
The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require.
5 Proven Patient Recruitment Strategies in Clinical Trials
We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Recently, four clinical research managers (crm) from merck (known.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Pharmaceutical companies have an opportunity to drive timelier recruitment.
Velocity Collaborates With Merck to Improve Diversity in Clinical
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Recently, four clinical research managers.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Awareness of challenges and reviewing strategies that can.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. The ability to move.
Global Clinical Trial Operations Merck Careers
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. The ability to.
Pharmaceutical Companies Have An Opportunity To Drive Timelier Recruitment By Working With Patients To Develop Clinical Trial.
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective.