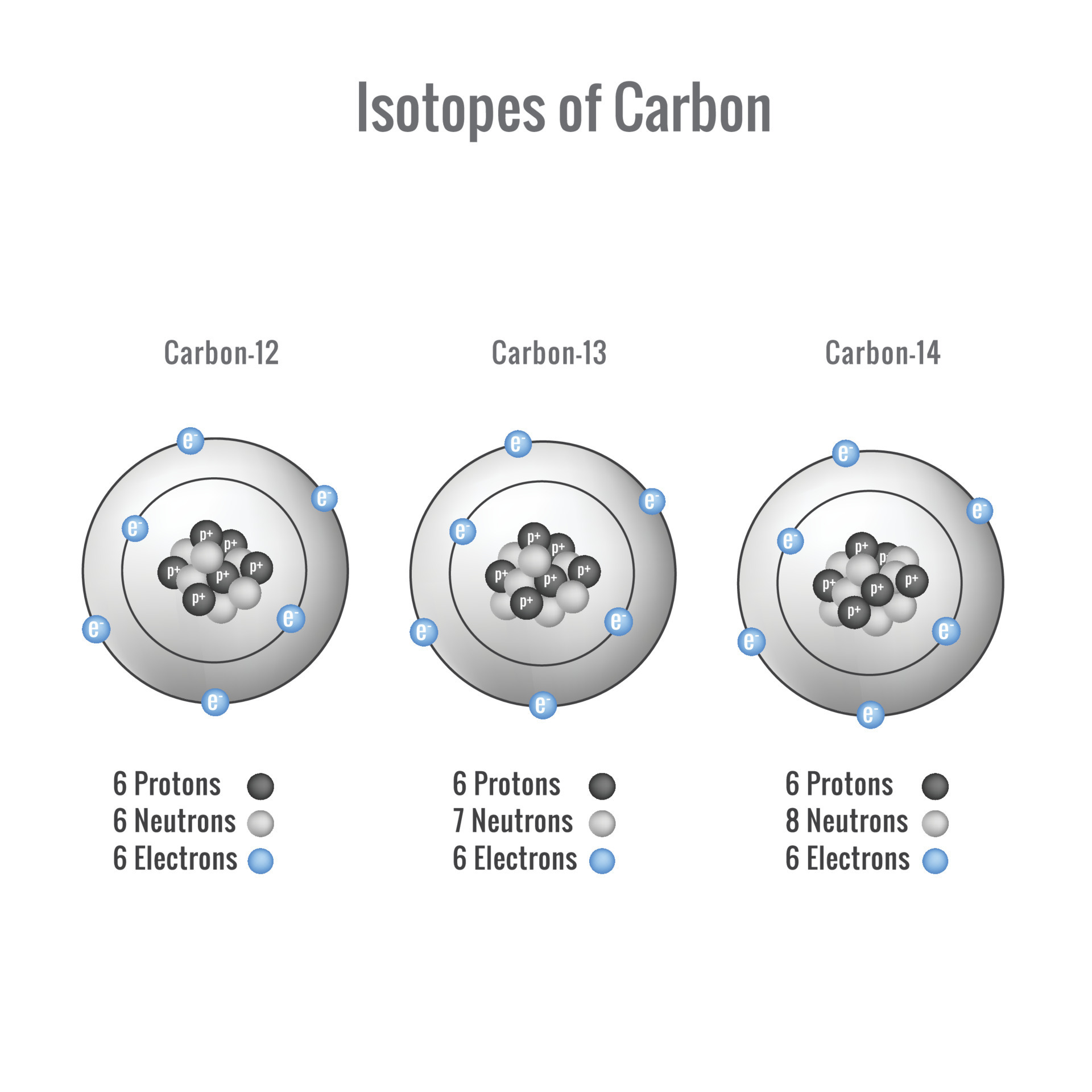
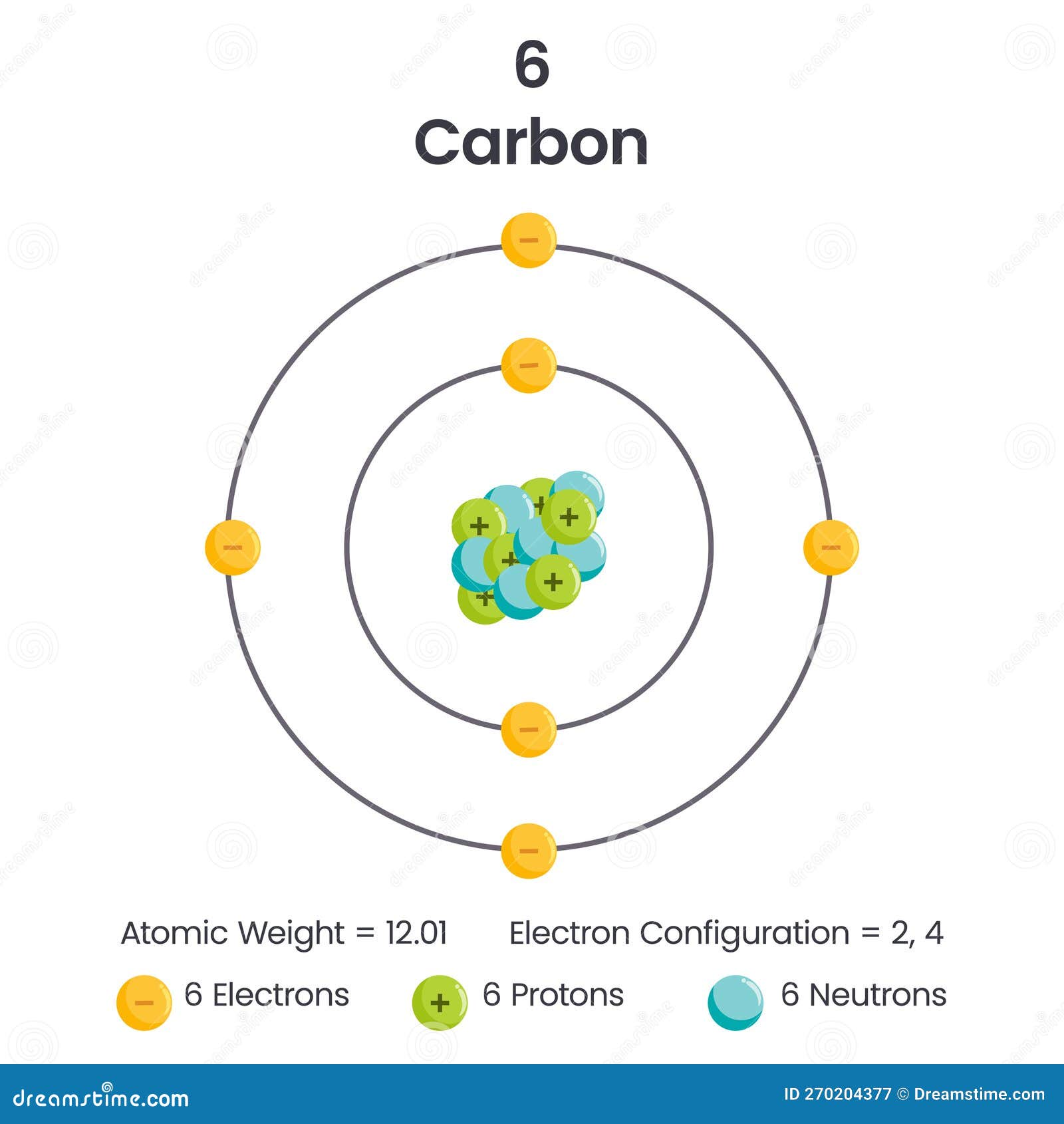
How Many Electrons Does Carbon Have Available For Chemical Bonding - Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds.
Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and.
Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds.
Carbon Atom Diagram
Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon (4 electrons in.
Carbon Dioxide Covalent Bond
Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through.
PPT Biochemistry PowerPoint Presentation, free download ID89333
Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon has four.
How Many Valence Electrons Does Carbon (C) Have?
Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through.
Electrons In Carbon
Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon is known to form almost ten million compounds, a large majority of all chemical.
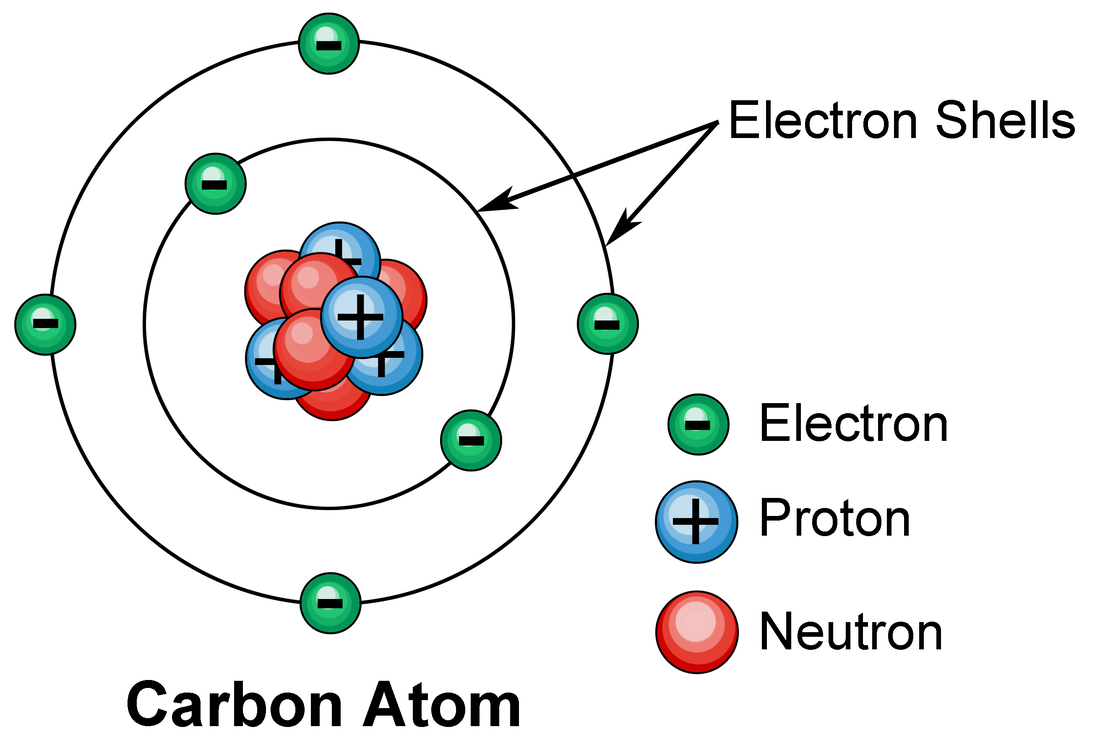
[Class 10 Chemistry] What is Carbon and its compounds? Teachoo
Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen,.
Covalent Bonding Diagram
Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon has four.
5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence
Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon (4 electrons in.
Carbon Protons Neutrons Electrons
Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon is known to form almost ten million compounds, a large majority of all chemical.
Carbon Element 6 Electron Configuration Vector Illustration Diagram
Carbon (4 electrons in the valence shell) combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons,. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen,.
Carbon (4 Electrons In The Valence Shell) Combines With Four Hydrogen Atoms To Form A Stable Covalent Compound Where It Shares 8 Electrons,.
Carbon atom electrons occupy specific orbitals, with six electrons in outer shells, forming bonds through covalent sharing, ionic. Carbon has four valence electrons, enabling it to form four covalent bonds with a variety of atoms, including hydrogen, oxygen, nitrogen, and. Carbon is known to form almost ten million compounds, a large majority of all chemical compounds.
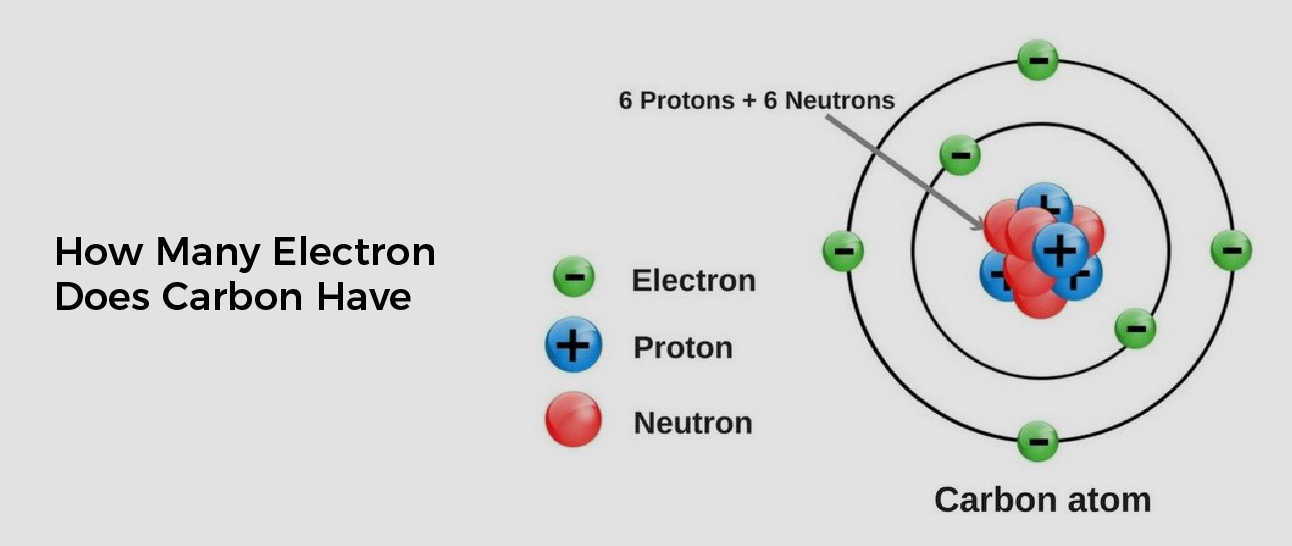


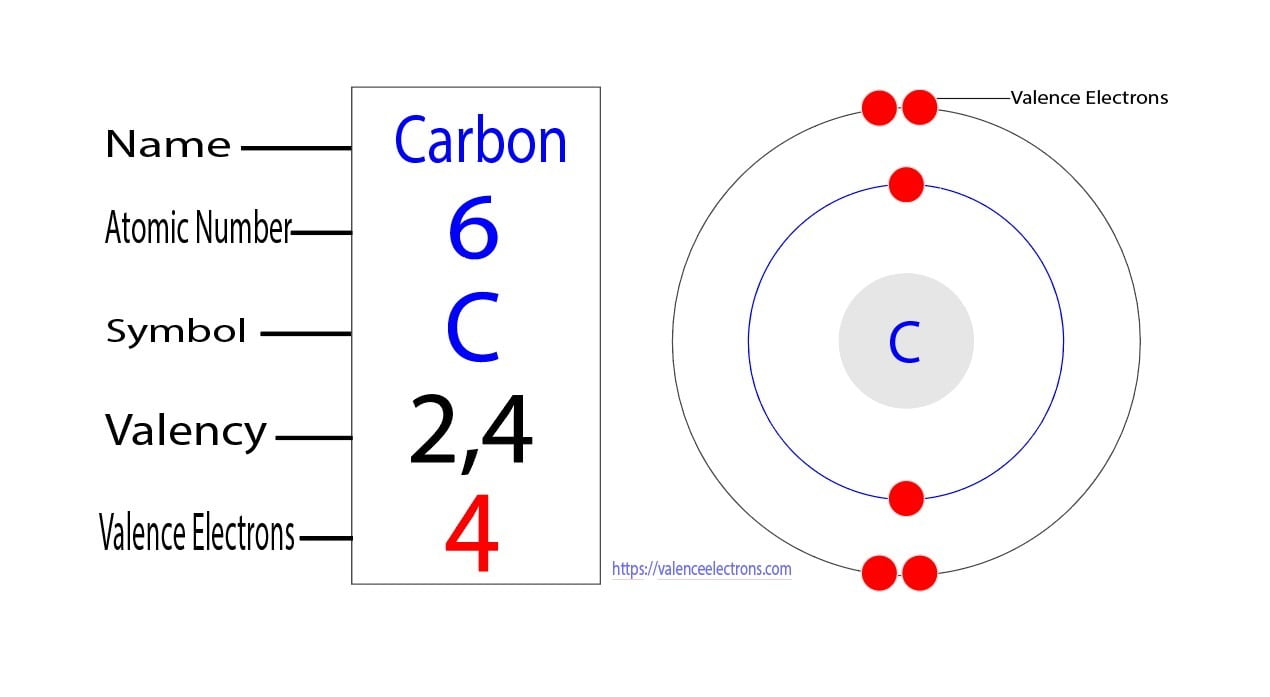
![[Class 10 Chemistry] What is Carbon and its compounds? Teachoo](https://d1avenlh0i1xmr.cloudfront.net/large/a0f30a5a-e7f4-4637-a158-8ca55cf3a17d/electronic-configuration-of-carbon-atom---teachoo.jpg)