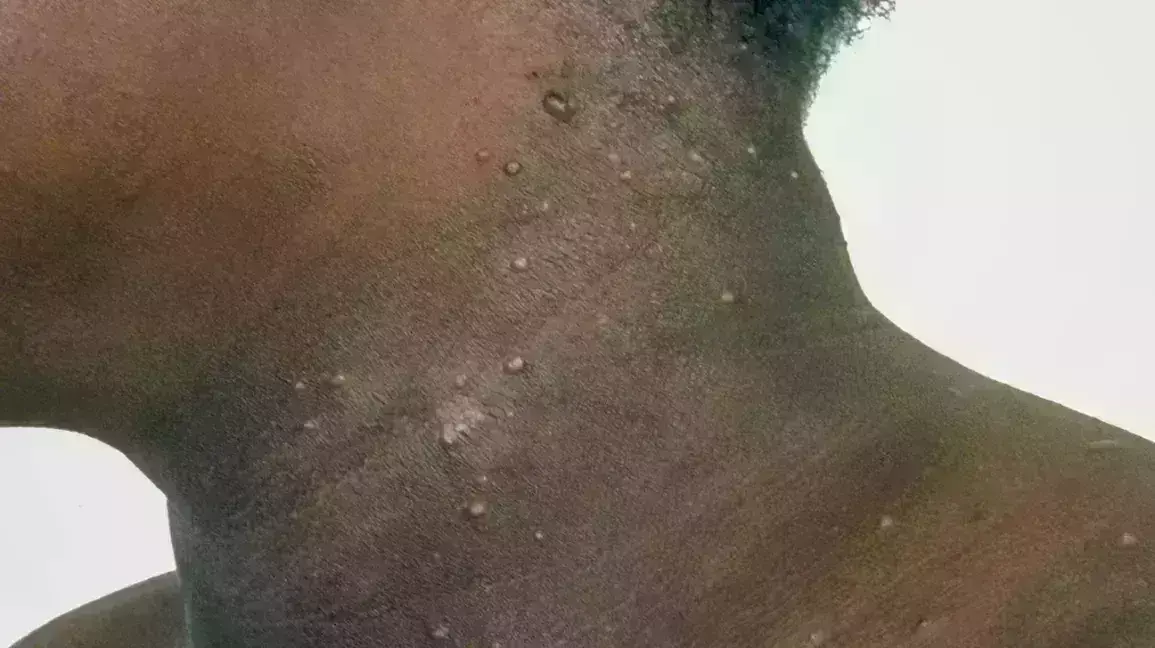
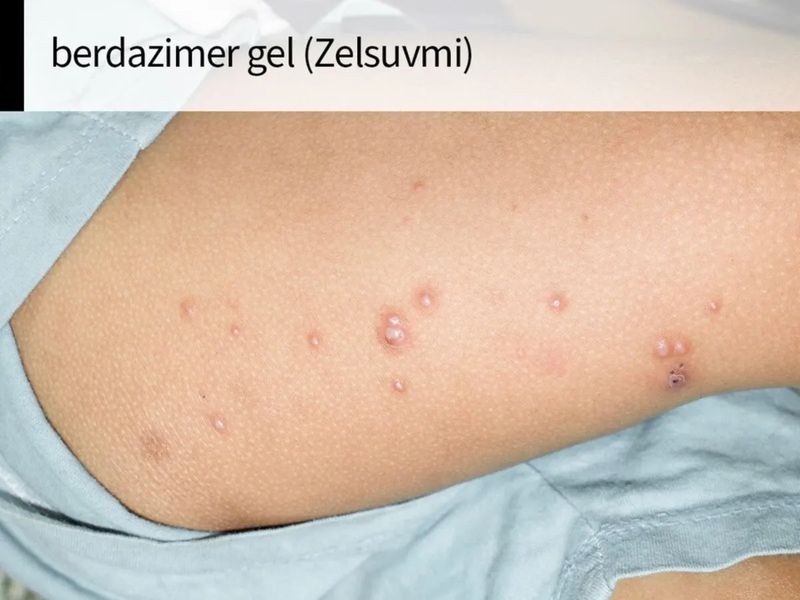
Berdazimer Gel Available In Usa - This medicine is available only with. Zelsuvmi is expected to be available in the united states in the second half of 2024. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi™ is expected to be commercially available during the second half of 2024.
Zelsuvmi is expected to be available in the united states in the second half of 2024. This medicine is available only with. There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). Zelsuvmi™ is expected to be commercially available during the second half of 2024. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric.
Zelsuvmi is expected to be available in the united states in the second half of 2024. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi™ is expected to be commercially available during the second half of 2024. This medicine is available only with. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric.
FDA Approves Berdazimer Gel A FirstofItsKind Treatment for
Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi is expected to be available in the united states in the second half of 2024. This medicine is available only with. Zelsuvmi™ is expected to be commercially available during the second half of.
FDA Approves Zelsuvmi (berdazimer topical gel) for the Treatment of
Zelsuvmi is expected to be available in the united states in the second half of 2024. There is currently no therapeutically equivalent version of zelsuvmi available in the united states. This medicine is available only with. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. Description berdazimer topical.
LAUNCH ALERT Berdazimer Topical Gel 10.3 (ZELSUVMI) Receives FDA
This medicine is available only with. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). Zelsuvmi™ is expected to be commercially available during the second half of 2024. There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi is expected to be available in the united states in the second half of.
FDA Approves Berdazimer Topical Gel as First Treatment for Molluscum
There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi is expected to be available in the united states in the second half of 2024. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in.
ZELSUVMIBerdazimerTopicalGel10.3.PrescribingInformationand
Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. Zelsuvmi is expected to be available in the united states in the second half of 2024. Zelsuvmi™ is expected to be commercially available during the second half of 2024. Description berdazimer topical gel is used to treat molluscum contagiosum.
FDA Approves Berdazimer Gel 10.3 for the Treatment of Molluscum
Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. This medicine is available only with. Zelsuvmi™ is expected to be commercially available during the second half of 2024. There is currently no therapeutically equivalent version of.
Topical berdazimer gel safe and effective therapy for molluscum
Zelsuvmi is expected to be available in the united states in the second half of 2024. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. Zelsuvmi™ is expected to be commercially available during the second half of 2024. Description berdazimer topical gel is used to treat molluscum contagiosum.
Efficacy and Safety of Topical Nitric Oxide−Releasing Berdazimer Gel in
Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. Zelsuvmi™ is expected to be commercially available during the second half of 2024. Zelsuvmi is expected to be available in the united states in the second half of 2024. There is currently no therapeutically equivalent version of zelsuvmi available.
FDA Approves Berdazimer Gel, 10.3 for the Treatment of Molluscum
There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. Zelsuvmi is expected to be available in the united states in the second half of 2024. This medicine is available only with. Description berdazimer topical.
DailyMed ZELSUVMI berdazimer kit
Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric. This medicine is available only with. Description berdazimer topical gel is used to treat molluscum contagiosum (molluscum bumps). There is currently no therapeutically equivalent version of zelsuvmi available in the united states. Zelsuvmi is expected to be available in.
Description Berdazimer Topical Gel Is Used To Treat Molluscum Contagiosum (Molluscum Bumps).
Zelsuvmi™ is expected to be commercially available during the second half of 2024. There is currently no therapeutically equivalent version of zelsuvmi available in the united states. This medicine is available only with. Zelsuvmi is a nitric oxide (no) releasing agent indicated for the topical treatment of molluscum contagiosum (mc) in adults and pediatric.
/medriva/media/post_banners/content/uploads/2024/01/fda-approval-of-berdazimer-gel-103-for-molluscum-contagiosum-20240106054011.jpg)



/medriva/media/post_banners/content/uploads/2024/01/berdazimer-gel-103-fda-approval-20240108194215.jpg)